This is why neck pain often occurs in the morning:
Technology for Vertebral Decompression is born
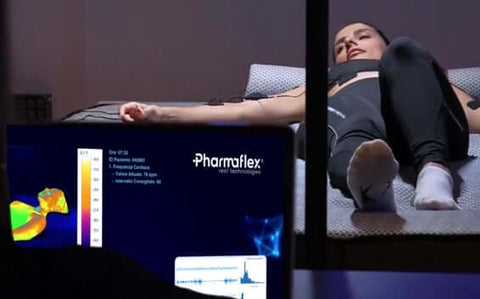
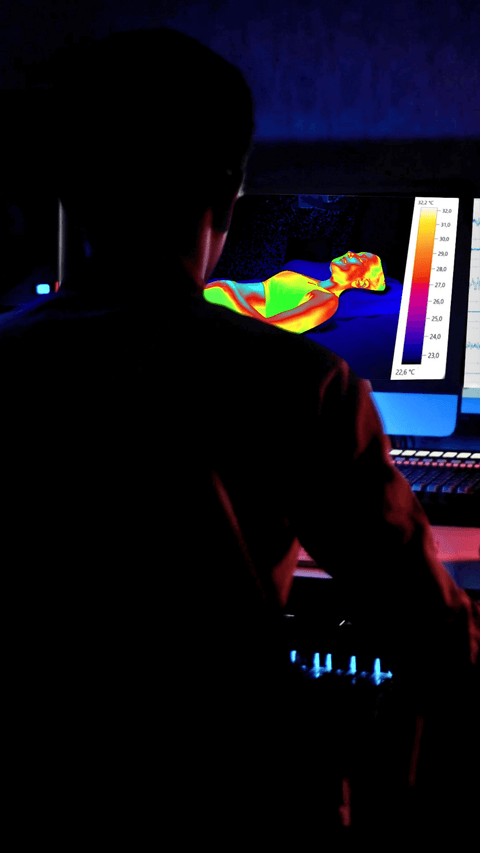
Pressure point analysis
This procedure measures how the body's weight is distributed over the resting surface. During the analysis, an individual lies on the product, and using advanced technology, the areas where the body exerts the most pressure are detected. This makes it possible to identify areas at risk of stress or discomfort, such as the shoulders, hips, or lower back.
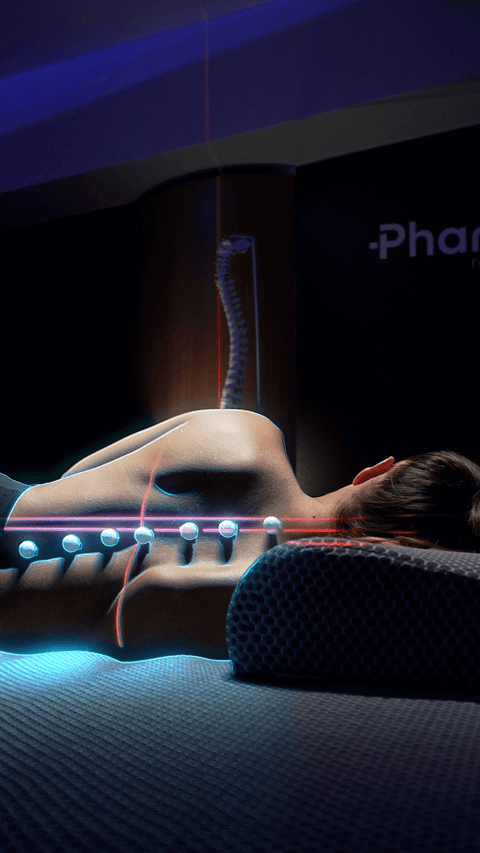
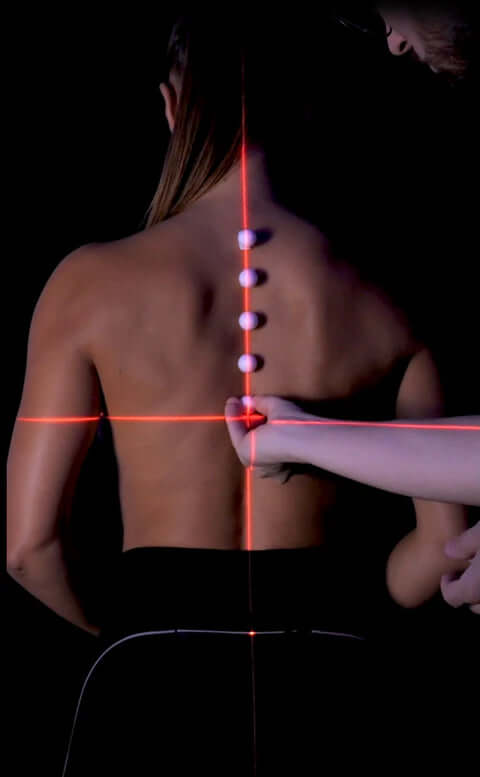
Spinal alignment
Through state-of-the-art instruments, we strive to monitor the proper alignment of the cervical spine in every resting position. The goal is to ensure that no conditions occur that could lead to muscular or skeletal decompensation.
Spinal decompression
This advanced high-precision test aims to monitor the spine before and after the use of our products. By comparing the results against other products, our biomedical engineers can accurately assess how effectively the action of the devices relieved compression on the discs and vertebrae.
Muscle relaxation
At this stage, thin electrodes are attached to the skin or inserted into the muscles to record the electrical signals generated during muscle contractions. This test is particularly important because it allows us to detect the actual ability of our products to reduce muscle tension and assess the quality of rest.
According to experts
The opinions of experts whom we asked to test and express an opinion.30 Days Free Return
2-year warranty
30 Days Free Return
100+ Pharmaflex Physiotherapists
We employ more than 100 physical therapists and health professionals to take care of our clients, from product design to post-purchase therapies|
|
Of course. Our products are Class 1 medical devices. Therefore, you can take a 19% deduction, when filing your income tax return, either in 730 or in Personal Income (formerly UNICO PF Form).
Certainly, the covers are unlined and conveniently washable. The padding, however, should not be washed. See the user's manual for more information on this.
- Tolerance of +/-2 cm on measurements.